A research study led by Teacher Sebyung Kang and Teacher Sung Ho Park in the Department of Biological Sciences at UNIST has actually revealed an amazing development in cancer treatment. The research study group has actually effectively established extraordinary “NK cell-engaging nanodrones” efficient in selectively targeting and removing cancer cells, using a prospective option for intractable kinds of cancers.
These groundbreaking NKeNDs all at once show cancer-targeting ligands, such as HER2Afb or EGFRAfb, and NK cell-recruiting ligands, aCD16Nb, on the surface area of the AaLS through the SpyCatcher/SpyTag protein ligation system. The double ligand-displaying NKeNDs, called HER2 @NKeND and EGFR@NKeND, have actually shown the capability to selectively bind to HER2-overexpressing SK-OV-3 cells and EGFR-overexpressing MDA-MB-468 cells, respectively, along with human NK cells.
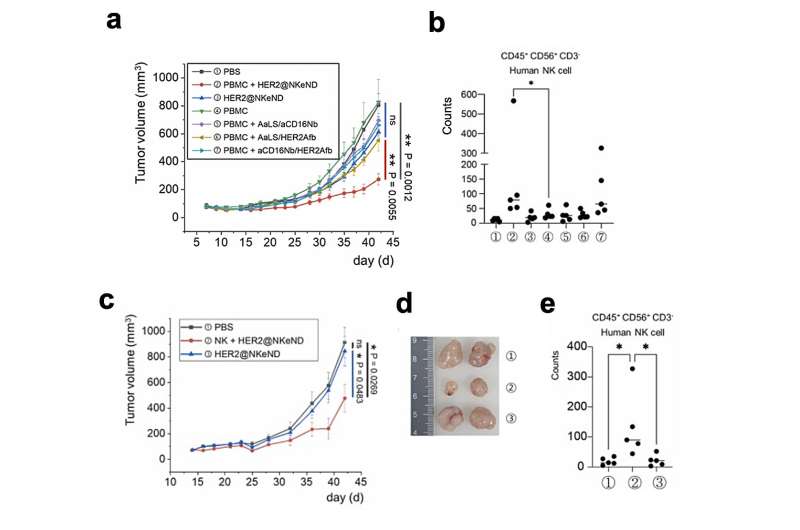
The physical engagement of human NK cells with the target cancer cells moderated by the NKeNDs triggers the NK cells, allowing them to get rid of the target cancer cells in vitro successfully. Extremely, in SK-OV-3 tumor-bearing mice, the administration of HER2 @NKeNDs together with human PBMCs assists in the seepage of triggered human NK cells into the growth websites. As an outcome, tumor development is substantially reduced without triggering visible negative effects.
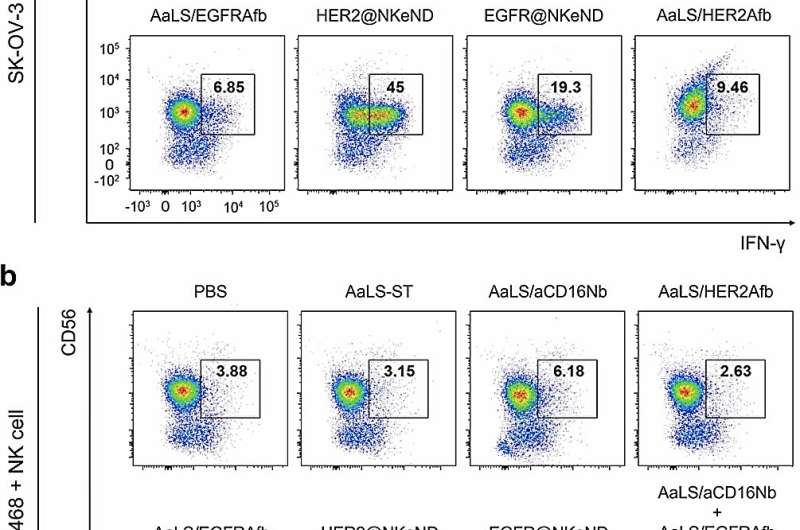
This research study showcases an unique technique to establishing cancer-specific NK cell engagers by making use of protein cage nanoparticles and recombinant cancer cell binders. It uses significant capacity for the selective treatment of formerly intractable kinds of cancers.
Teacher Kang Se-byung revealed his enjoyment about the research study, mentioning, “This research study provides brand-new possibilities for immune treatment through NK cell shipment nanodrones, getting rid of obstacles such as the motion and survival of NK cells We intend to offer brand-new chances for personalized treatments that selectively attend to numerous kinds of cancer through more research study, consisting of cancer-specific immune cell induction.”
The research study is released in the journal Nano Today